Introduction:The development of a tool to predict the sensitivity of MDS patients to azacytidine has great clinical applicability. Furthermore, a tool that is able to dynamically predict response to azacytidine while on therapy would be of utility to the clinician who must determine when the efficacy of the drug has been lost.
Goal: The purpose of this work was to evaluate clinical and laboratory parameters to develop markers of ineffective hematopoiesis that correlate with the development of resistance to azacytidine in MDS patients.
Methods: We conducted a retrospective cohort study of all patients with MDS with Int-2/hi risk IPSS score treated with 5-azacytidine at the London Health Science Centre between January 2008 and December 2014. The study was approved by the institutional Research Ethics Board. Relevant data, including bone marrow aspirate and biopsy, cytogenetics, peripheral blood counts, transfusion frequency, and azacytidine dosing and administration were extracted from the electronic patient record. Response to azacytidine, as assessed by IWG 2006 criteria, was recorded and time to treatment failure was calculated. The parameter representative of ineffective erythropoiesis was derived using an ROC curve generated by comparing the RDW during time periods of low red blood cell transfusion burden to time periods of high transfusion burden. Parameters representative of ineffective hematopoiesis in each of the megakaryocytic and granulocytic lineages were derived using ROC curves generated by comparing changes in each of the respective counts during time periods with effective erythropoiesis and known azacytidine treatment response (based on bone marrow blast counts and cytogenetic profile), and time periods with effective erythropoiesis and proven treatment failure. The parameter for ineffective erythropoiesis was defined as hemoglobin < 110 mg/L with RDW < 18. The parameter for ineffective megakaryopoiesis was defined to be platelet count less than 70 ∙ 109 cells/mL or platelet count drop over 2 cycles of azacytidine greater than 70 ∙ 109 cells/mL. The parameter for ineffective granulopoiesis was defined as absolute neutrophil count less than 1.3 ∙ 109 cells/mL or absolute neutrophil count drop over 2 cycles of azacytidine greater than 1.3 ∙ 109 cells/mL.
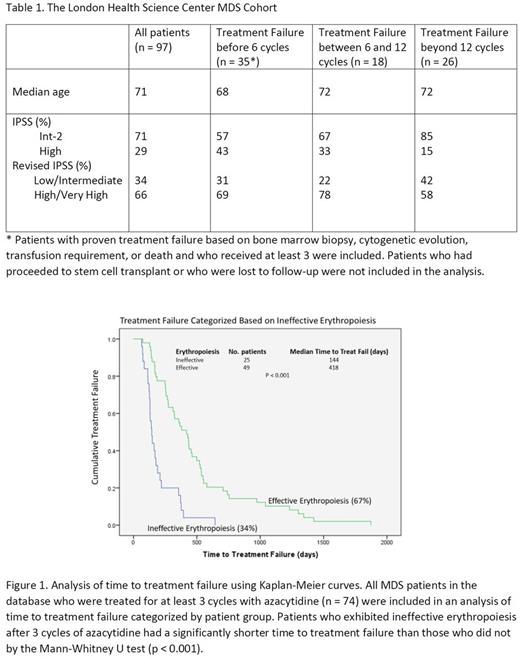
Results: The characteristics of all MDS patients included in the cohort is summarized in Table 1. There was a total of 97 patients in the cohort, with 74 patients receiving greater than 3 treatment cycles and having sufficient response data for the derivation of hematopoietic parameters. The presence of ineffective erythropoiesis after the 3rd cycle of azacytidine had a significant correlation with the time to treatment failure (Figure 1). The median time to treatment failure of MDS patients was 144 days for patients with ineffective erythropoiesis after 3 cycles of azacytidine, while it was 418 days for patients with effective erythropoiesis. The parameters for ineffective megakaryopoiesis and ineffective granulopoiesis after 3 cycles of azacytidine had no correlation with time to treatment failure. There were 25 patients who attained response to azacytidine beyond 6 cycles and who had proven treatment failure based on either increasing blast count or cytogenetic evolution along with sufficient peripheral blood count data corresponding to the time of treatment failure. Among these patients, the development of ineffective granulopoiesis followed by the development of ineffective megakaryopoiesis had a positive predictive value of 87% for disease progression.
Conclusion: The presence of ineffective erythropoiesis after 3 cycles of azacytidine was the strongest predictor of azacytidine treatment failure in Int-2/Hi IPSS MDS patients. The earliest peripheral blood markers of disease progression after initial response reflect the development of ineffective granulopoiesis followed by ineffective megakaryopoiesis, with effective erythropoiesis preserved.
Lazo-Langner: Daiichi Sankyo: Research Funding; Alexion: Research Funding; Bayer: Honoraria; Pfizer: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal